St. Joseph’s Institute for Autoimmune Diseases director Dr. Bob Lahita argues Democratic presidential candidate Robert F. Kennedy Jr. is spreading misinformation and disinformation on vaccines on ‘Cavuto: Coast to Coast.’
The Centers for Disease Control and Prevention said Thursday that it is recommending a new immunization this fall to protect infants under eight months and some older babies at increased risk of severe illness from respiratory syncytial virus.
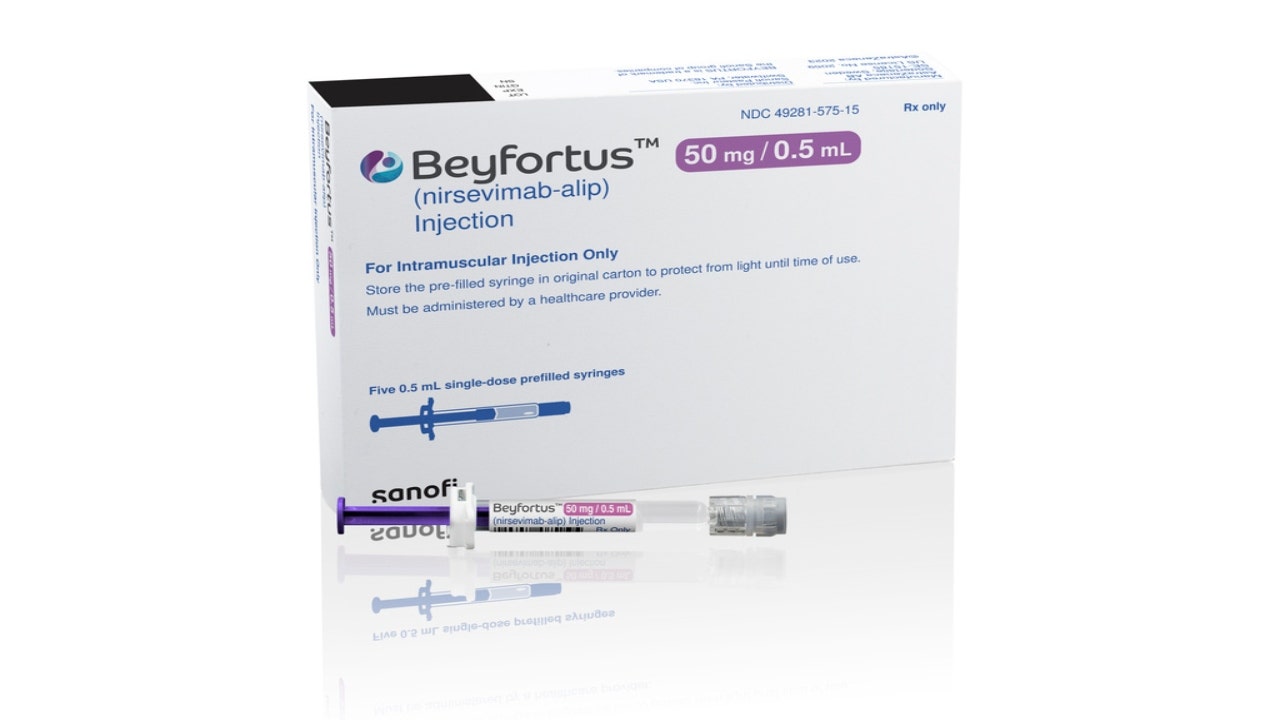
The agency said Director Dr. Mandy Cohen adopted an advisory committee’s recommendation for the use of nirsevimab, a long-acting monoclonal antibody product developed by AstraZeneca and Sanofi that has been shown to reduce the risk of hospitalizations and healthcare visits for infants by around 80%.
The CDC noted that antibodies are key to fighting off infections, explaining that monoclonal antibodies are manmade proteins that mimic the antibodies that are naturally produced.
“Making this immunization available means that babies will be able to receive antibodies to prevent severe RSV disease, providing a critical tool to protect against a virus that is the leading cause of hospitalization among infants in the U.S.,” it said in a statement.
TORNADO DAMAGE TO NORTH CAROLINA PFIZER PLANT UNLIKELY TO CAUSE SIGNIFICANT DRUG SUPPLY IMPACTS, FDA SAYS

On Thursday, Aug. 3, 2023, a panel of outside advisers to the Centers for Disease Control and Prevention recommended that babies get the drug to protect them against a respiratory virus that sends tens of thousands of American children to the hospita (AstraZeneca via AP / AP Newsroom)
RSV is one of the most common causes of childhood respiratory illness, and an estimated 58,000 to 80,000 children under the age of five are hospitalized annually across the country due to infection.
As many as 300 children younger than five years of age die due to RSV every year.
The CDC recommends a dose of the drug for all infants younger than eight months born during or entering their first RSV season when a baby is most at risk for severe illness. That season typically extends from fall to spring.
GET FOX BUSINESS ON THE GO BY CLICKING HERE

House Ways and Means Committee hearing on Capitol Hill November 3, 2015, in Washington, D.C. (Mark Wilson/Getty Images / Getty Images)
For a smaller group of children between eight and 19 months who are at increased risk of severe RSV disease, a dose is recommended in their second season.
“As we head into respiratory virus season this fall, it’s important to use these new tools available to help prevent severe RSV illness,” Cohen said. “I encourage parents of infants to talk to their pediatricians about this new immunization and the importance of preventing severe RSV.”
Although the new drug is not a vaccine, the panel notably also supported including it in Vaccines for Children, a government program providing free immunizations.

A patient suffering from respiratory syncytial virus is lying in a hospital bed in a pediatric ward of the Olgahospital of the Klinkum Stuttgart. (Marijan Murat/picture alliance via Getty Images / Getty Images)
CLICK HERE TO READ MORE ON FOX BUSINESS
Nirsevimab, administered as an injection, was approved last month by the U.S. Food and Drug Administration. It is expected to be available in the fall and sold under the brand name Beyfortus. The Associated Press reported that it is anticipated to cost $495 per dose and to be covered by insurance.
In late June, then-Director Dr. Rochelle Walensky endorsed the committee’s recommendations for the use of RSV vaccines from GlaxoSmithKline and Pfizer for seniors, using shared clinical decision-making.
The Associated Press contributed to this report.







